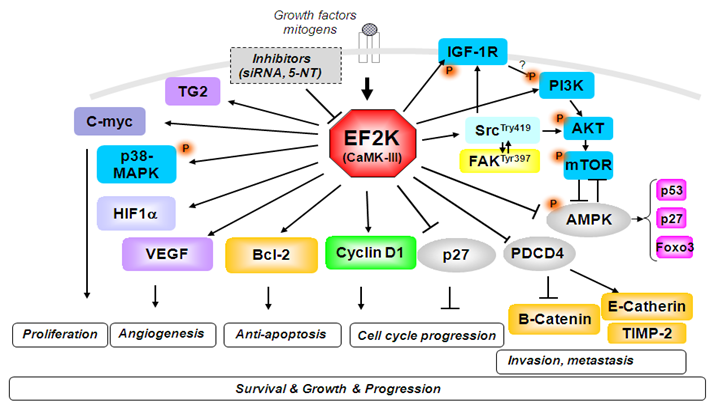
Figure 1. eEF2K induces activity of multiple and clinically significant oncogenic signaling pathways in solid cancer cells.
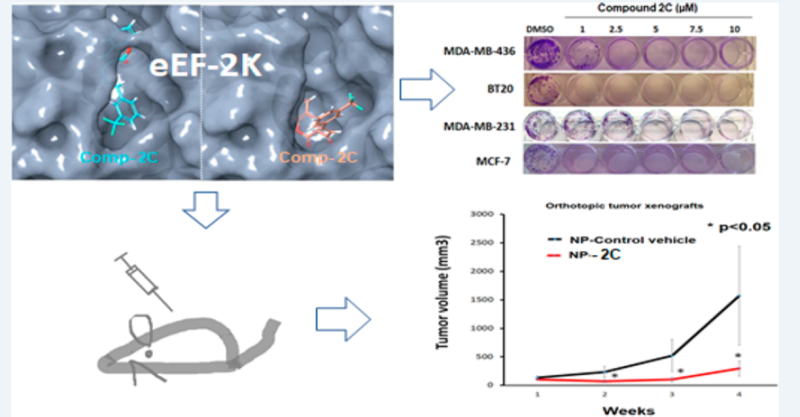
Figure 2. Development of highly-specific inhibitors of eEF2K-Kinase signaling for treatment of patients with eEF2K-overexpressing cancers (Onder 2021). A novel eEF2K inhibitor suppresses tumor growth in mice bearing TNBC tumor xenografts.
Identification of novel oncogenic molecular drivers, therapeutic targets and development of targeted cancer therapeutics such as small molecule inhibitors and RNA-based nanotherapeutics for aggressive solid cancers.
After validation of oncogenic eEF2-Kinase (EF2K) kinase as one of the major driver of triple negative breast cancer (TNBC), lung, pancreatic, and ovarian cancers (Figure 1 Tekedereli 2012, Ashour 2014, Hamurcu 2016, Bircan 2018, Asik 2018, Karakas 2023, Onder 2021 -2023 ), in collaboration with a group of multidisciplinary team of scientists with expertise in computational chemistry, synesthetic and medicinal chemistry, cancer biology, genetics and applied mathematics, we designed and synthesized novel (patented) inhibitors (Figure 2) and miRNA-based therapeutics (Figure 3) for targeting eEF2K with significant in vivo anti-tumor efficacy and safety in multiple tumor models.
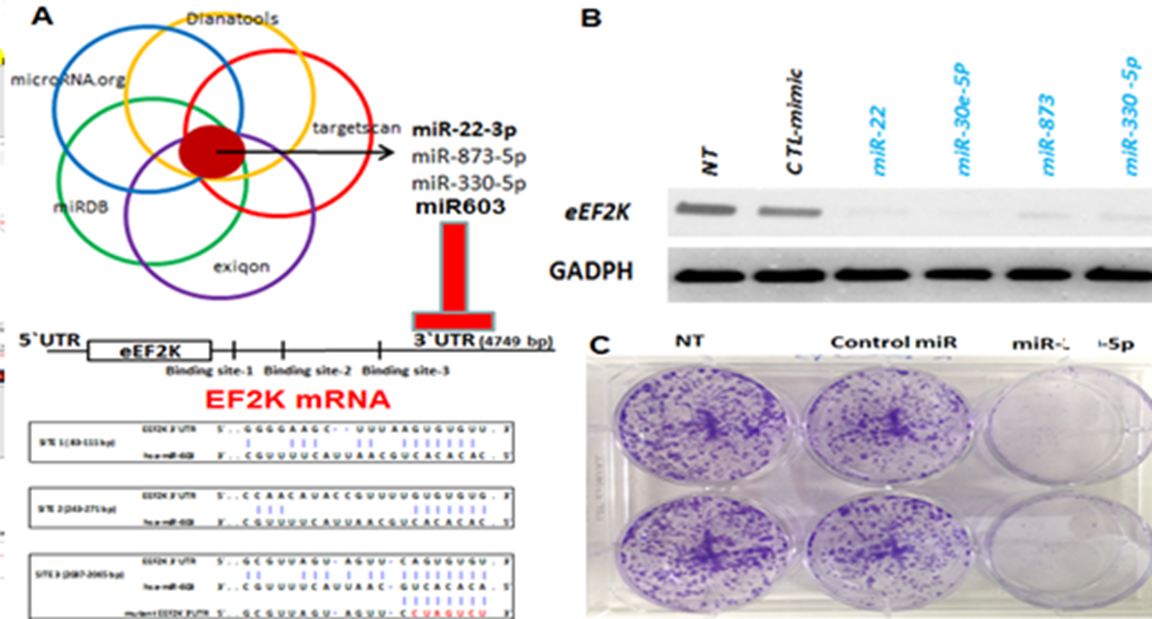
Figure 2. Development of highly specific inhibitors of eEF2K-Kinase signaling for treatment of patients with eEF2K overexpressing cancers (Onder 2021). A novel eEF2K inhibitor suppresses tumor growth in mice-beating TNBC tumor xenografts.
Figure 3. Non-protein coding RNAs such as microRNAs may have oncogenic or tumor suppressor function in tumor cells and they are involved in various cellular processes in normal and cancer cells. Their expression is dysregulated in cancer cells and tumor suppressor miRNA (Red) expressions are lost/reduced and oncogenic miRNAs (green) are upregulated in cancer cells.
Tumor suppressor microRNA (miRNA)-based nanotherapeutics for the treatment of aggressive solid tumors (i.e, TNBC, pancreatic, ovarian, lung, colorectal cancers ( Bayraktar 2017, Asik 2018, Gorur 2022,)
Development of nanotechnology-based delivery platforms for delivery of cancer therapeutics such as RNA-therapeutics (miRNAs) and small molecule inhibitors into tumors and tumor-microenvironment.
Nanotechnology/nanotherapeutics provide an invaluable solution to overcome biological barriers such as poor bioavailability and pharmacokinetics and weak cellular uptake and reduce side effects due to distribution of drugs in normal tissues(Ozpolat 2010,2014, Mohklis &Ozpolat 2019, Kara&Ozpolat 2021). Some solid tumors have physiological barriers that limits reaching of cancer therapeutics and RNA-based therapeutics, from reaching tumor cells (Kara &Ozpolat 2022). Leaky nature of tumor angiogenic vessels nanoparticles NPs passively accumulate in tumor but not in normal tissues thus, delivery cancer -based therapeutic cargo by NPs can target broader tumor cell populations and may significantly enhance or maximize antitumor efficacy against hard-to-treat cancers (Gurbuz&Ozpolat 2019, Kara &Ozpolat 2022). We aim to develop highly effective and safe cancer nanotherapeutics and nanodrugs using lipids-based nanodelivery vehicles (i.e, nanoliposomes, immunoliposomes, micelles), polymer-based nanoparticles (ie. chitosan, albumin) and magnetic or metal-based nanocarriers (i.e, Iron-Feoxide, Cobalt, Gold and Silver) for the delivery of various novel cancer therapeutics (i.e, small molecule inhibitors, microRNA, non-coding RNA, RNA or oligonucletide-based therapeutics, therapeutic peptides and chemical small molecule inhibitors
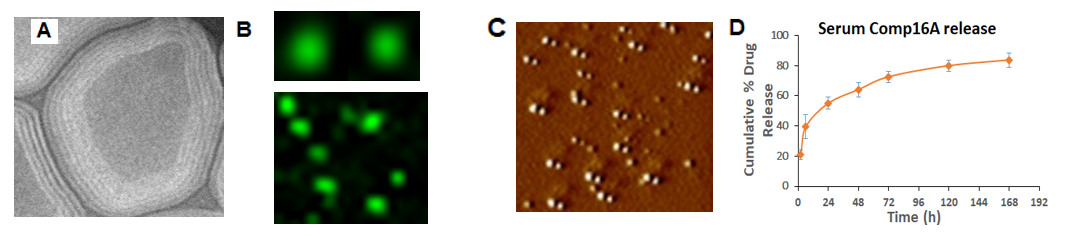
Figure 4. Characterization of novel slow-release long-acting Single-Lipid-based Nanoparticles (SLNPs) incorporating EF2K inhibitor compound that we developed. A) Transmission electron microscopy (TEM) shows multi-lamellar lipid structures, B) by fluorescent microscopy of Comp16A (Green)-loaded SLNPs. C) Atomic force microscopy (AFM) indicates a highly homogenoussize distribution of SLNP-Comp-16A in 3D-view with average 55nm diameter. D) Slow drug release by SLNP in serum and time-dependent release kinetics in serum (37C). Compound was measured by UV spectrometry in serum up to 166 h after removing SLNPs by centrifugation.
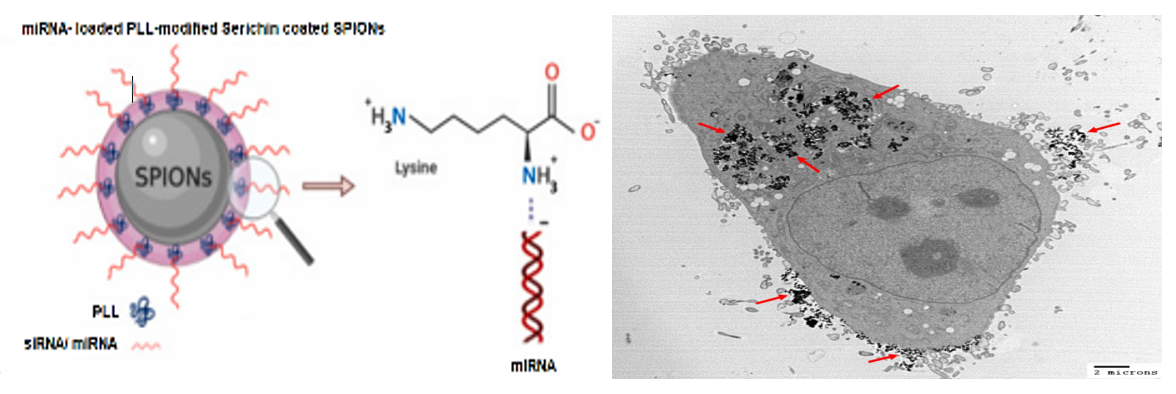
Figure 5. Schematic of supermagnetic Iron-oxide-based Nanocarriers(SPIONs) (please see our review Kara& Ozpolat 2024) conjugated and loaded with microRNA are internalized by MDA-MB-231 breast cancer cells capured by Transmission Electron Microscopy. Black dots indiceted with red arrows)
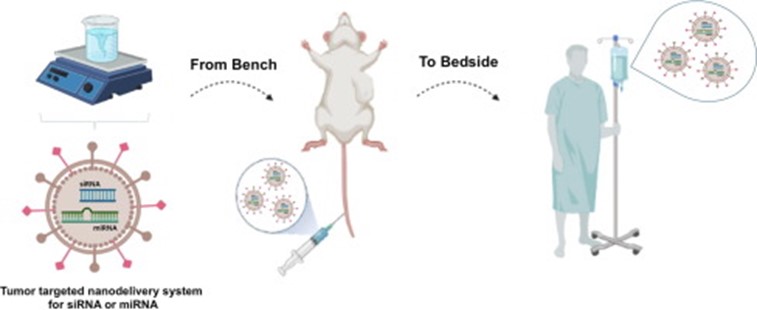
Figure 6. Preclinical development of microRNA-nanotherapeutics and their testing in mice bearing humor tumor xenografts and translation of the human clinical trials.
Development of therapeutic strategies targeting K-RAS oncogenic signaling for targeted pancreatic cancer therapy. We aim to develop next generation of highly targeted nanotherapeutics for inactivating mutated KRAS, which is a major oncogenic driver of 30% of human cancers ) or overactivated KRAS/MAPK signaling in cancer patients by utilizing small molecule inhibitors (patented) which showed significant antitumor efficacy in pancreatic cancer and other KRAS driven cancer models in mice with no sign of toxicity.
Identification drug-resistance mechanism to anti-estrogen therapies and development of novel targeted therapies to overcome resistance in hormone (estrogen) receptor positive (ER+) breast cancer. About 70% of breast cancer patients have estrogen receptor positive cancer and use anti-estrogen hormonal therapy (tamoxifen faslodex or aromatze inhibotorsetc). However some patienst develop resistante to anti-estrogen therapies and needed to be treated with chemotheray or other drugs ( See our recent review on the topic, Ozyurt& Ozpolat 2023) To adress thi issue we started a new project to target resistance tumors and identified several potential molecular targets to reverse to resistance or inhibit them as a novel therapy for hormon therapy resiistant cancers.
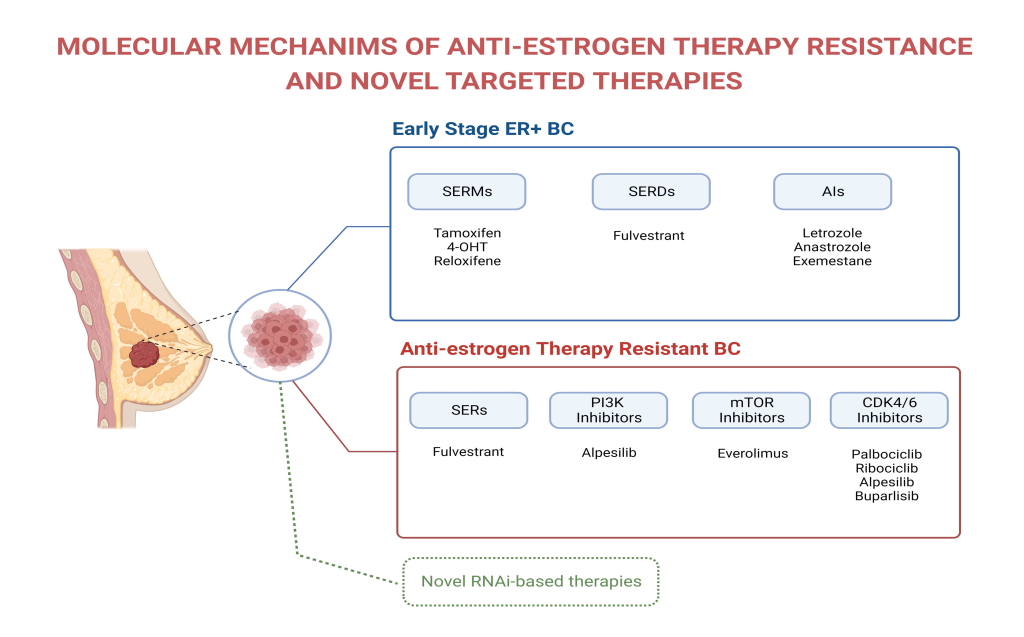
Figure 6. Preclinical development of microRNA-nanotherapeutics and their testing in mice bearing humor tumor xenografts and translation of the human clinical trials.
Development of therapeutic strategies targeting K-RAS oncogenic signaling for targeted pancreatic cancer therapy. We aim to develop next generation of highly targeted nanotherapeutics for inactivating mutated KRAS, which is a major oncogenic driver of 30% of human cancers ) or overactivated KRAS/MAPK signaling in cancer patients by utilizing small molecule inhibitors (patented) which showed significant antitumor efficacy in pancreatic cancer and other KRAS driven cancer models in mice with no sign of toxicity.
